Quasi-Drugs
What are Quasi-Drugs?
- The term “Quasi-Drugs” means items (goods constituting medicines shall be excluded herefrom) designated by the Minister of Food and Drug Safety (hereinafter
referred to as the Minister of MFDS), falling under any of the following:
- A. Fibers, rubber products or other similar products intended to be used for treating, reducing, or preventing diseases in human and animals;
- B. Non-equipment or non-machinery products that have minimal effect or do not have a direct effect on human, or other similar products;
- C. Products intended to be used for disinfection, insecticide or other similar purposes to prevent infectious diseases. >
※ Classification of quasi-drugs into three groups pursuant to the ‘Designation of the Scope of Quasi-Drugs (Notice of Ministry of Food and Drug
Safety)
| Item Category? | Remark? | ||
|---|---|---|---|
| Group 1 | A. Sanitary Pad | 1) Menstrual Pad | Fibers, rubber or similar products used for sanitary purposes |
| 2) Menstrual Tampon | |||
| B. Mask | 1) Surgical mask | ||
| 2) Dust Mask | |||
| C. Sanitary products used for preservation, protection and treatment etc. of the affected area? | 1) Eye Bandage | ||
| 2) Bandage | |||
| 3) Elastic bandage | |||
| 4) Plaster bandage | |||
| 5) Cylindrical elastic bandage (Stokinet) | |||
| 6) Absorbent Gauze | |||
| 7) Absorbent Cotton | |||
| 8) Adhesive Plaster | |||
| D. Wet wipes for oral hygiene | |||
| E. Other similar products | |||
| Group 2 | A. Odor inhibitors such as bad breath (halitosis) eliminators | 1) Mouth fresheners (for internal use and mouthrinses) | Products that have minimal effect or do not have a direct effect on human |
| 2) Antiperspirants (for external use only) | |||
| 3) Products for heat rash and skin erosion treatment | |||
| 4) Toothpastes | |||
| 5) Bath products (for external use only) | |||
| B. Extirpators, inhibitors, repellents and insect-attracting pesticides for flies and mosquitoes, etc.? | |||
| - extirpators, inhibitors, insect-attracting pesticides and repellents | |||
| C. Contact Lens Care Products | |||
| D. Products which do not contain nicotine, falling under the following | 1) Products aiding in reducing the urge to smoke | ||
| 2) Products aiding in improving smoking habits | |||
| E. Externally applied disinfectants used directly on human? | |||
| (Main ingredients: hydrogen peroxide, isopropyl alcohol, benzalkonium chloride and cresol, or ethanol) | |||
| F. Ointment, cataplasma and anti-inflammatory/pain relief spray prescribed by the Manufacturing Standards | |||
| - Ointment, cataplasma and anti-inflammatory/pain relief spray | |||
| G. Products for internal use | 1) Low-vitamin and low- mineral products prescribed by the Manufacturing Standards of Quasi-drugs | ||
| 2) Energy drinks prescribed by the Manufacturing Standards of Quasi-drugs (liquid products for internal use) | |||
| 3) Digestive remedies for improving digestive health prescribed by the Manufacturing Standards of Quasi-drugs (liquid product for internal use), and intestinal drugs (solid product for internal use) | |||
| H. Products used for oral hygiene, etc. | 1) Externally applied liquid products for cleaning and disinfecting root canal | ||
| 2) Products used for correcting thumb sucking habits of infants and children | |||
| 3) Snoring aids (supplementary products) | |||
| 4) Teeth whitening products (containing ≤ 3% of hydrogen peroxide) | |||
| 5) Products used for cleaning or disinfecting false teeth (dentures) and braces | |||
| 6) Products used for dying dental plaque | |||
| Group 3 | A. Products used for disease prevention | 1) Insecticides | Disinfectants and insecticides used to prevent infectious diseases, antiseptics for public health and sanitation |
| 2) Rodenticides | |||
| B. Disinfectants not directly applied to human | 1) Disinfectants such as alcohols, aldehydes, cresol and soap | ||
| 2) Other products used for disease control | |||
Registration of manufacture or import business
- Any person who files an application for approval or notification of a quasi-drug product for the first time shall register as a quasi-drug manufacturer or import
business.
* To the commissioner of the competent Regional Office of Food and Drug Safety (RFDS)
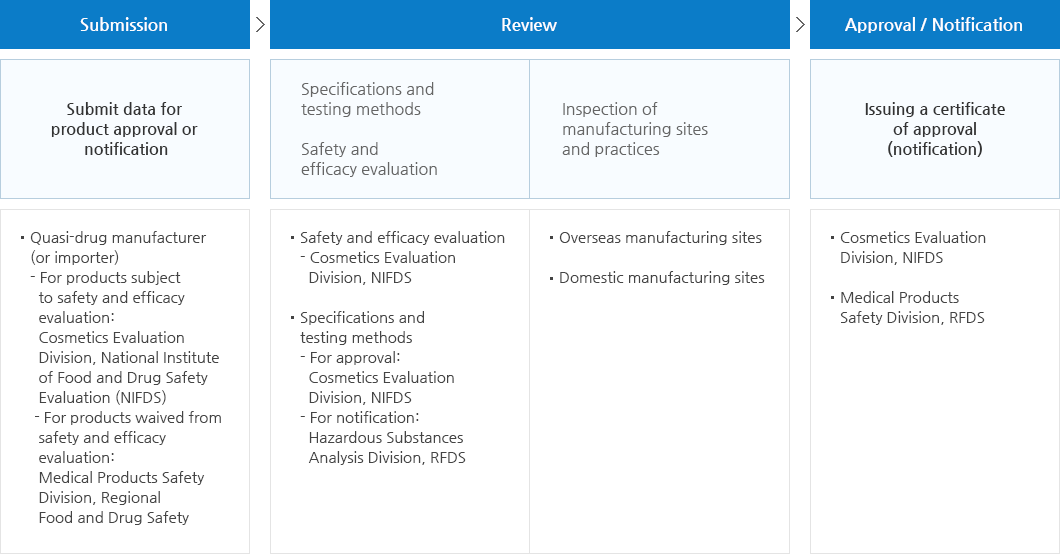
Procedures for manufacturing (or import) approval (or notification) of Quasi-Drugs
Items subject to approval or notification
- A. Items subject to notification
- Products listed in the Korean Pharmacopoeia or compendia and other pharmaceutical compendia recognized by the Minister of Food and Drug Safety (Any pharmaceutical not approved in Korea shall be excluded).
- Products for which specifications and test methods are prescribed by the Minister of MFDS
- Products in compliance with the Manufacturing Standards of Quasi-drugs officially notified by the Minister of MFDS.
- B. Items subject to approval
- Subject to approval by MFDS
- Quasi-drugs subject to safety and efficacy evaluation;
- When a new excipient that has not been used domestically is added to the product;
- Disinfectants and insecticides used for prevention of infectious diseases (Group 3).
- Subject to approval by RFDS
- Products that, in terms of active ingredients, specifications and volume (concentration for liquid forms), formulation, efficacy, usage and quantity, are same as those that have been approved and waived from safety and efficacy evaluation
Data requirements for approval or notification of Quasi-Drugs
- Data requirements for approval
- Data for safety and efficacy evaluation
- ① Origin or discovery and development history;
- ② Specifications and test methods;
- ③ Stability data (long-term or accelerated tests);
- ④ Toxicity data;
- ⑤ Efficacy and effectiveness data;
- ⑥ Current use in foreign countries;
- ⑦ Comparative review with other similar products domestically manufactured; and data on the characteristics of the product
- Data for specifications and testing methods
- ① Origin or discovery and development history;
- ② Structure identification, physical, chemical and biological characteristics (Data concerning the quality of product);
- Raw materials - Finished products - ③ Current use in foreign countries;
- ④ Comparative review with other similar products domestically manufactured; and data on the characteristics of the product
- B. Data requirements for notification
- ① Data demonstrating the item is subject to notification;
- ② Specifications and test methods.
- Common requirements
- Data for safety and efficacy evaluation
- Imported products: A certificate showing that the product is manufactured and marketed pursuant to the law of the corresponding country
- Ointments and cataplasma for external use that are in compliance with the Manufacturing Standards of Quasi-drugs notified by the Minister of MFDS and solid or liquid products for internal use: data necessary for inspection of good manufacturing practices (GMP)
Relevant regulations
- A. Laws
- Pharmaceutical Affairs Act
- Enforcement Decree of the Pharmaceutical Affairs Act
- Decree on Standards for Facilities of Drug Manufacturers and Importers
- Enforcement Rule of the Decree on Standards for Facilities of Drug Manufacturers and Importers
- Regulation on Safety of Pharmaceuticals
Relevant regulations
- B. Notices and published rulings
- Designation of the Scope of Quasi-Drugs
- Regulations on Qausi-drugs Approval, Notification and Review
- Regulations on Classifications of Quasi-Drugs
- Korean Pharmacopoeia (KP)
- Manufacturing Standards of Quasi-Drugs
- Korean Quasi-Drug Codex (KQC)
- Regulations on the Fees for Approval, etc. of Drugs, etc.
- Designation, Specifications and Test Methods for Tar Color Additives in Drugs, etc.
Processing period and fees
- Fees and processing period for approval (or notification) of items are as follows:
| Category? | Processing period? | Numbers of classified devices? | ||
|---|---|---|---|---|
| Online? | By mail or in Online person? | |||
| Approval | Quasi-Drugs - Approval - Specifications | 70 Days | 695,400 KRW | 768,600 KRW |
| and Test Methods - Safety and Efficacy | ||||
| Quasi-Drugs - Approval - Specifications | 55 Days | 308,750 KRW | 341,250 KRW | |
| and Test Methods | ||||
| Notification | Quasi-Drugs - Notification | 10 Days | 76,950 KRW | 85,050 KRW |
| Quasi-Drugs - Notification | 40 Days | 308,750 KRW | 341,250 KRW | |
| - Specifications and Test Methods | ||||
| Quasi-Drugs - Notification - Specifications and Test Methods - GMP | 90 Days | 926,250 KRW | 1,023,750 KRW | |
- The above described represents general cases of application for approval and notification of quasi-drugs. For more detailed information on fees and processing periods, please refer to the ‘Regulations on the Fees for Approval etc. of Drugs, etc.’ and the ‘Rules on Safety of Pharmaceuticals'