Functional Cosmetics
What are Functional Cosmetics?
- Cosmetics with functions, defined in the Cosmetics Acr, such as aiding in skin whitening, improving skin wrinkles, etc.
 Zoom
Zoom
- Skin
- Products aiding in the whiteening of the skin
- Products aiding in improving wrinkles in the skin
- Products aiding in tanning skin gently or protecting skin from ultraviolet rays
- Products aiding in alleviation acne breakouts
- Products aiding in alleviation dryness caused by atopic-prone skin
- Products aiding in thinning red lines caused by strecth marks
- Hair
- Hair-dye(Exclugin temporary dyes)
- Bleaching or dye-removing products
- Products aiding in alleviating hair loss symptoms(Products that increase hair volume by physical means are excluding)
- Body hair removal products(Excluding products that remove hair physically)
Who Should Request the Evaluation of Functional Cosmetics?
- With regards to the functional cosmetics, a Responsible Seller who intends to manufacture or sell functional cosmetics by manufacturing or importing them shall undergo an evaluation by the Minister of the Ministry of Food and Drug Safety (MFDS) or shall submit a report to the Minister of the MFDS for safety and effectiveness of each product pursuant to Article 4 of the Cosmetics Act (Examination, etc. of Functional Cosmetics). The same shall apply to any revision to the previously evaluated matters.
Evaluation of Functional Cosmetics
Subject and Examination Procedures
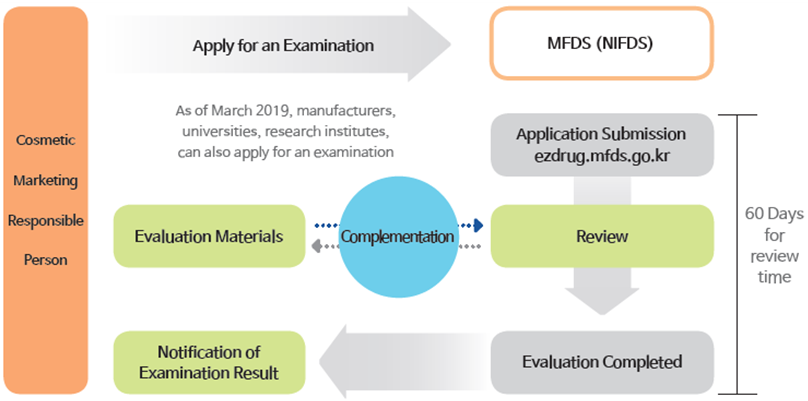 Zoom
Zoom
- Cosmetic Marketing Responsible Person
- Apply for an Examination
- As of March 2019, manufacturers, universities, research institues, can also apply for an examination
- Evaluation Materials
- Complementation
- Notification of Examination Result
- MFDS(NIFDS)
- Application Submission ezdrug.mfds.go.kr
- Review
- Evaluation Completed
- 60Days for review time
Data Requirement
- Data that verify safety, effectiveness, or function
- Data concerning the origin and details of R&D
- Data concerning the safety
- Data that verify effectiveness, or function
- Sun Protection Factor(SPF),waterproof Sun Protection Factor(SPF), and Protection Factor of UV-A(PA) data
- Data concerning the standards and test methods (including samples)
Relevant Government Department
- National Institute of Food and Drug Safety Evaluation (NIFDS), Cosmetics Evaluation Division
Deadline and Fees
| Types | Fees | No. of days | |
|---|---|---|---|
| Electronic civil petitions | By mail, in-person, | ||
| 1. Request for evaluation of functional cosmetics | 189,000 KRW | 210,000 KRW | 60 |
| 2. Evaluation of revision to functional cosmetics | |||
| A. With regards to the specifications of raw materials, if a revision is made to the test methods, efficacy and effect (excluding the cases in which submission of data that verify effectiveness or function is omitted), or standards and test methods (excluding pH and methanol) | 51,000 KRW | 57,000 KRW | 60 |
| B. Revisions other than those in Section A. | 25,000 KRW | Coronary stent | 15 |
| 3. Application for reissuance of notice of examination results for functional cosmetics | 1,800 KRW | 2,000 KRW | 7 |
Subject Exempt from Evaluation
Flowchart for the Evaluation of Functional Cosmetics
- Items with the same active ingredients, functions, applications, forms, specifications and test methods as ones notified by the MFDS(KFCC)
- Items with the same active ingredients, functions, applications, forms, specifications and test methods as previously approved ones
Relevant Government Department
- National Institute of Food and Drug Safety Evaluation (NIFDS), Cosmetics Evaluation Division
Deadline and Fees
- No fees are required for report on functional cosmetics
- Reports are usually processed on the same day. However, if there are supplementations, or the application is submitted after office hours, there may be a delay.

