Summary of Pharmaceutical (Herbal Medicinal Preparations) Approval
Pharmaceutical(Herbal Medicinal Preparations) Approval
- (Overview) Approve pharmaceuticals whose safety, efficacy, and quality are identified (including pharmaceutical ingredients) through technological review and inspection for their manufacturing and distribution.¹
| Data Requirements for Approval |
|---|
|
(New Drug)
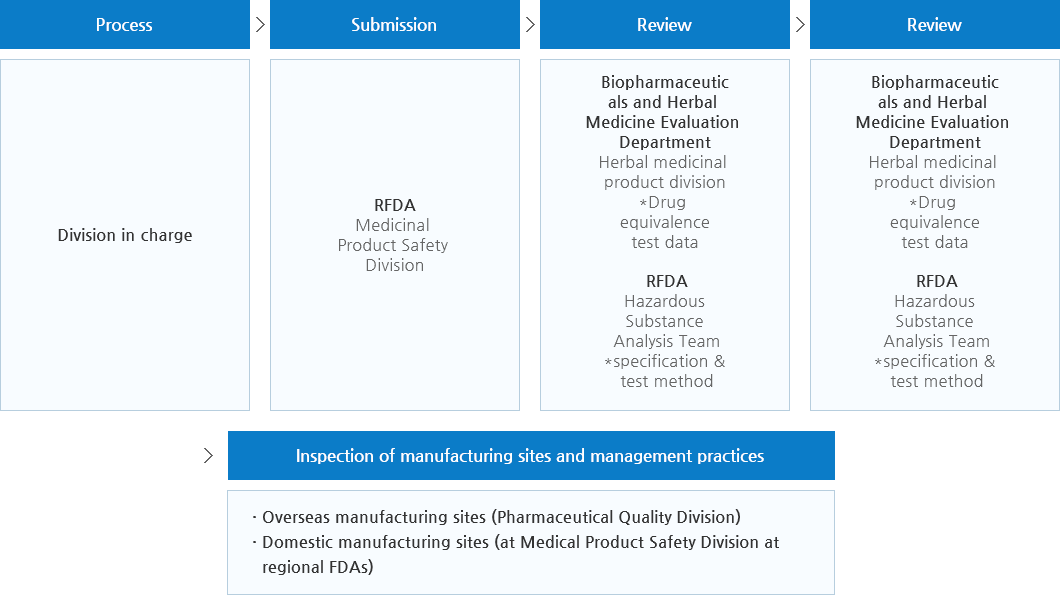
- (Review by Drug Evaluation Department, Biopharmaceuticals and Herbal Medicine Evaluation Department) Safety & efficacy data, specifications & test methods, Drug Master Files (DMF), certificate of manufacturing and marketing (Imported Pharmaceutical) Data such as name and address of manufacturers of active pharmaceutical ingredients - (Review by Other Departments) Evaluation data of conducting of Good Manufacturing Practice (GMP)
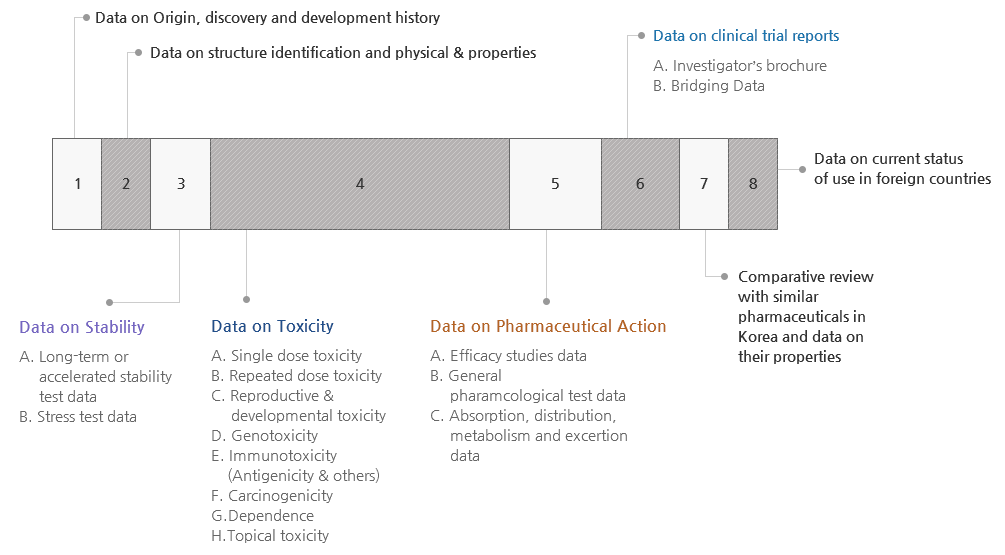
Data for Specifications & Test Method Review
- A. Data on pharmaceutical ingredients
- Data on structure identification
- Data on physical & chemical properties
- Data on manufacturing methods
- Data on specifications & test methods are stated
- Supportive data on specifications & test methods
- Data on test results
- Data on reference standards, reagents, and test solutions
- Data on containers & packaging materials
- B. Data on drug product
- Data on composition
- Data on manufacturing methods
- Data on specifications & test methods are stated
- Supportive data on specifications & test methods
- Data on test results
- Data on reference standards, reagents, and test solutions
- Data on containers & packaging materials
- (Pharmaceutical required for data submission) Selectively submit data required for safety and efficacy evaluation among submission data for new drug
- (Generic Drug)? Submit bioequivalence test data and quality data instead of safety and efficacy data such as toxicity, pharmacology, clinical trials Generic drugs are reviewed by Drug Evaluation Department and approved by regional FDAs
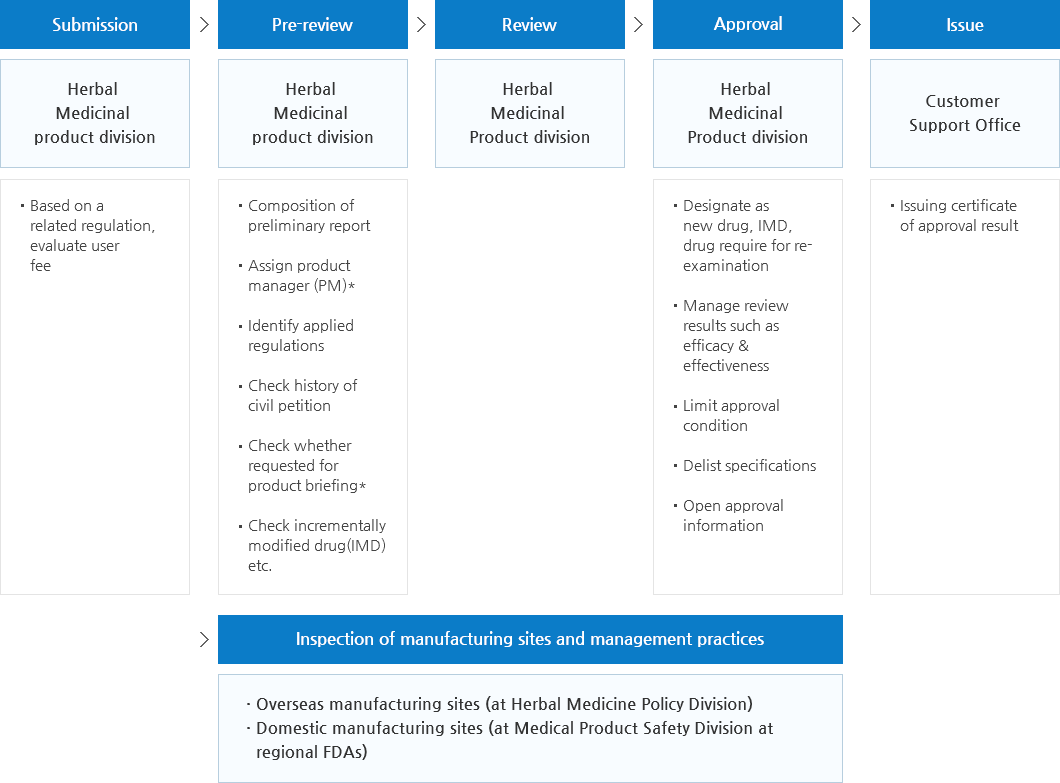
Work Flow for Approval of Herbal Drug Products(TKMP, HMP)
- (New drug, Pharmaceutical required for data submission)
- Generic Drug
- In the whole process of drug review and approval, consult with Central Pharmaceutical Affairs Advisory Committee (CPAC), if necessary
- 「Pharmaceutical Affairs Act」Article 31
- 「Pharmaceutical Affairs Act」Article 2 Subparagraph 8
- Regulation on Pharmaceuticals Approval, Notification and Review (MFDS Notification) Article 2 Subparagraph 8, The regulation on the approval & registration of herbal drug products(TKMP, HMP) (MFDS Notification) Article 2 Subparagraph 6.
- 「Regulation on Safety of Pharmaceuticals」(Ordinance of the Prime Ministerial) Article 9
- 「Regulation on Safety of Pharmaceuticals」(Ordinance of the Prime Ministerial) Article 4 (1) 3