Overview
What is Global Innovative product on Fast Track(GIFT)?
- The GIFT means a supporting program for accelerating regulatory review of "global innovative products", in order to facilitate market launch of innovative medicinal products intended for treatment of life-threatening or serious diseases and assure faster supply of such products to patients.
Key benefits of GIFT
- For GIFT-designated products, the period of regulatory review process can be shortened to 75% of usual review time. Additionally, the following regulatory supports are provided:
- A product for expedited review is designated in the early stage of clinical development.
- It is allowed to submit some data not directly related with the product's safety after product approval.
- Global review standard, including those provided in the ICH guidelines, are concurrently applied in order to avoid potential regulatory conflicts with foreign regulatory authorities. Such concurrent application of internationally harmonized requirements to regulatory review and authorization of innovative products will be helpful for expedited product approval and market launch in oversea markets.
- Rolling review process is applied which enables regulatory review on prepared and available information and data first.
- Regulatory supports, including RA (regulatory affairs) consulting and review schedule, are provided through close communication between the regulatory reviewer and the developing company, such as product briefing and explanation of supplementation.
Scope of products for GIFT designation
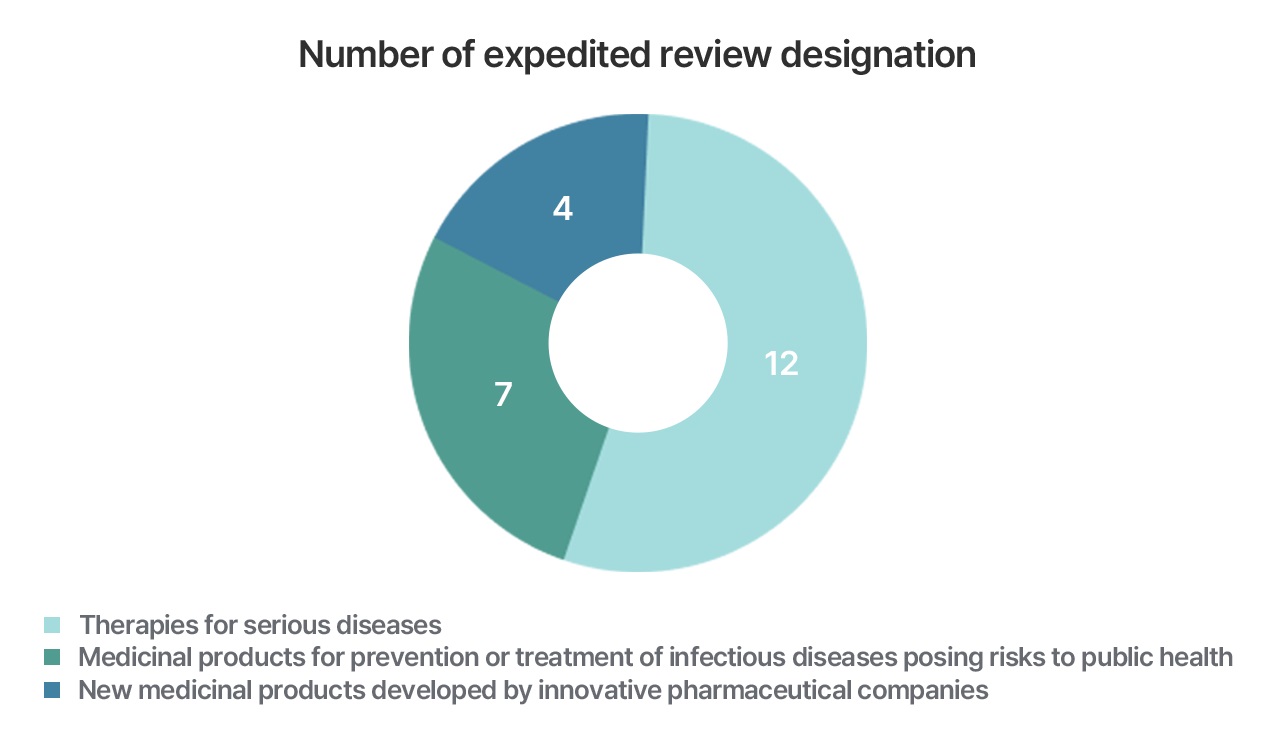
- A medicinal product intended for treatment of life-threatening or serious diseases, such as cancers, or rare diseases, without existing treatment or with a significant improvement in efficacy compared to the existing treatment.
- A medicinal product intended for prevention or treatment of infectious diseases with a serious threat to public health, such as an infectious disease outbreak caused by bioterrorism or pandemic (including outbreak of infectious disease with a significant concern of pandemic), without existing treatment or with a significant improvement in efficacy compared to the existing treatment, or a novel product with completely different mode of action/mechanism from existing treatments.
- A new medicinal product developed by an innovative pharmaceutical company designated and notified by the Ministry of Health and Welfare.
- A combination of a medical device and a medicinal product designated for expedited review.
- (Legal basis) Article 35-4 (Priority Review Designation) of the Pharmaceutical Affairs Act, Article 40-2 (Priority Review Designation) of the Rules of the Safety of Pharmaceuticals and Article 7 (Priority Review) of the Special Act on the Promotion of Development and Emergency Supply of Medical Products for Public Health Emergency Preparedness.
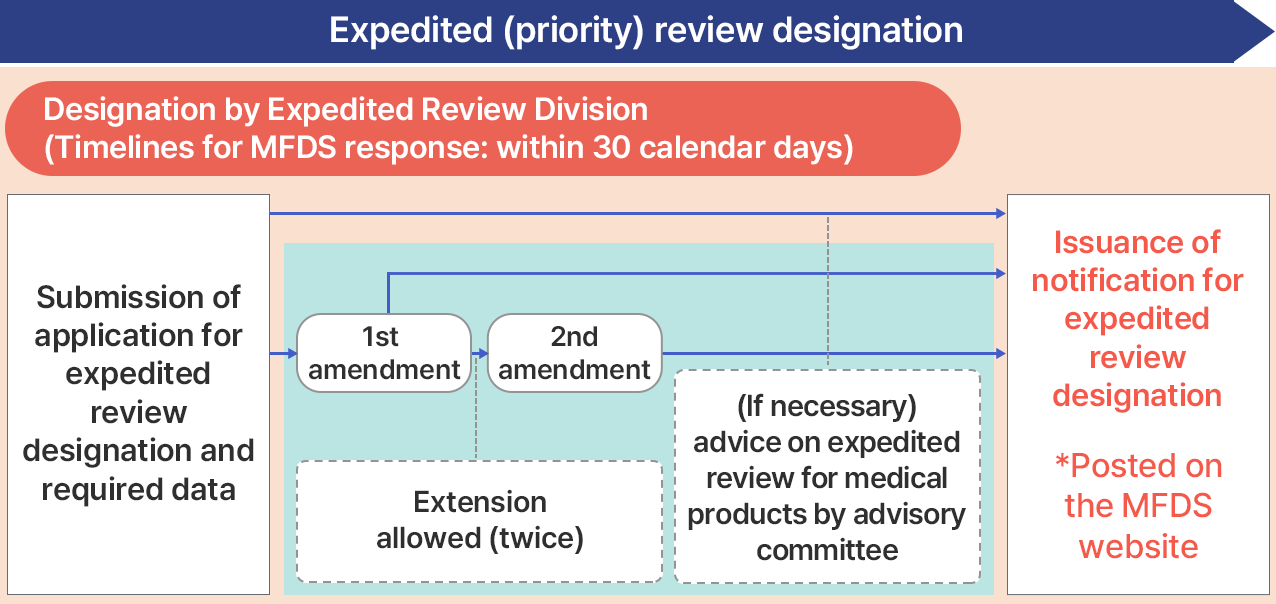
GIFT designation application process and submissions
Notes
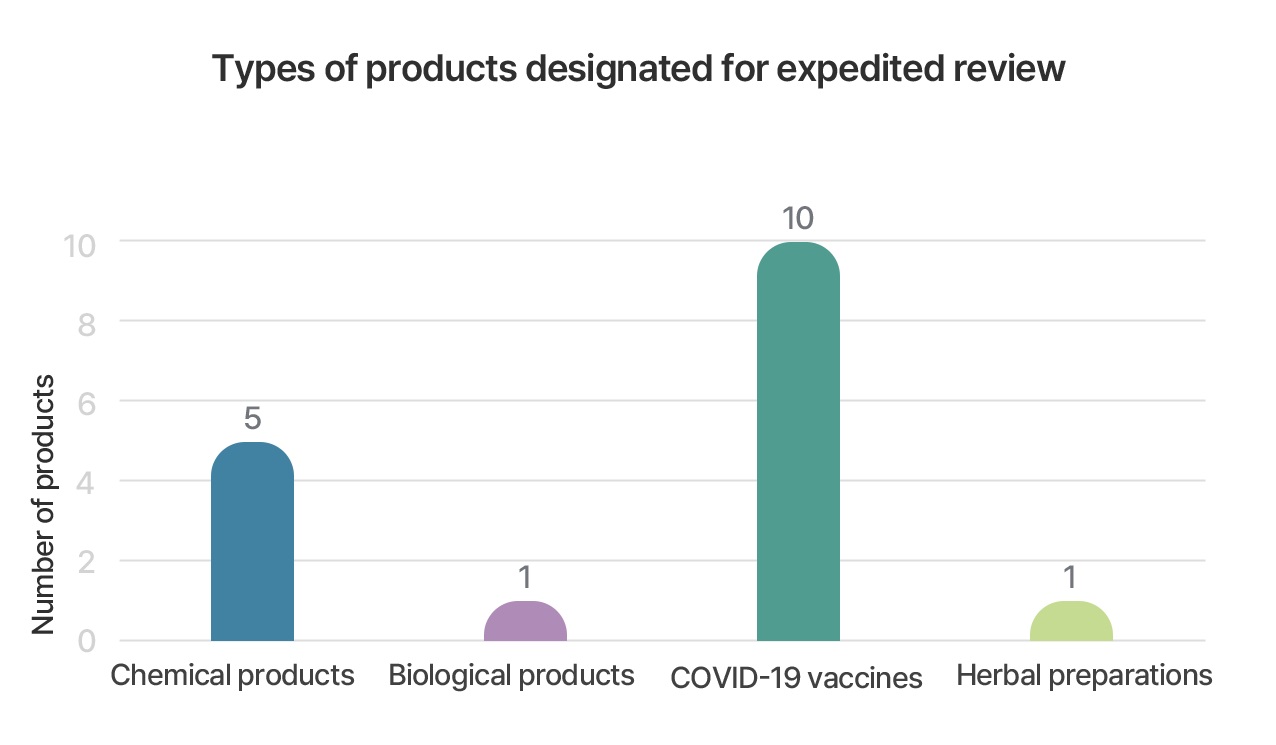
- Medicinal products designated for expedited (priority) review include biologics, recombinant products, Korean traditional medicines, and herbal preparations.
- If rapid introduction of certain products is needed, such as medicinal products used in the pandemic of infectious disease, these products can be designated for expedited review and the Expedited (Priority) Review Designation Notice can be issued, even when the designation application is not submitted. In addition, the expedited review designation and regulatory review process can be concurrently conducted.
- For a new medicinal product developed by an innovative pharmaceutical company, the expedited review designation application can be submitted at the same time of submission of the product approval application.
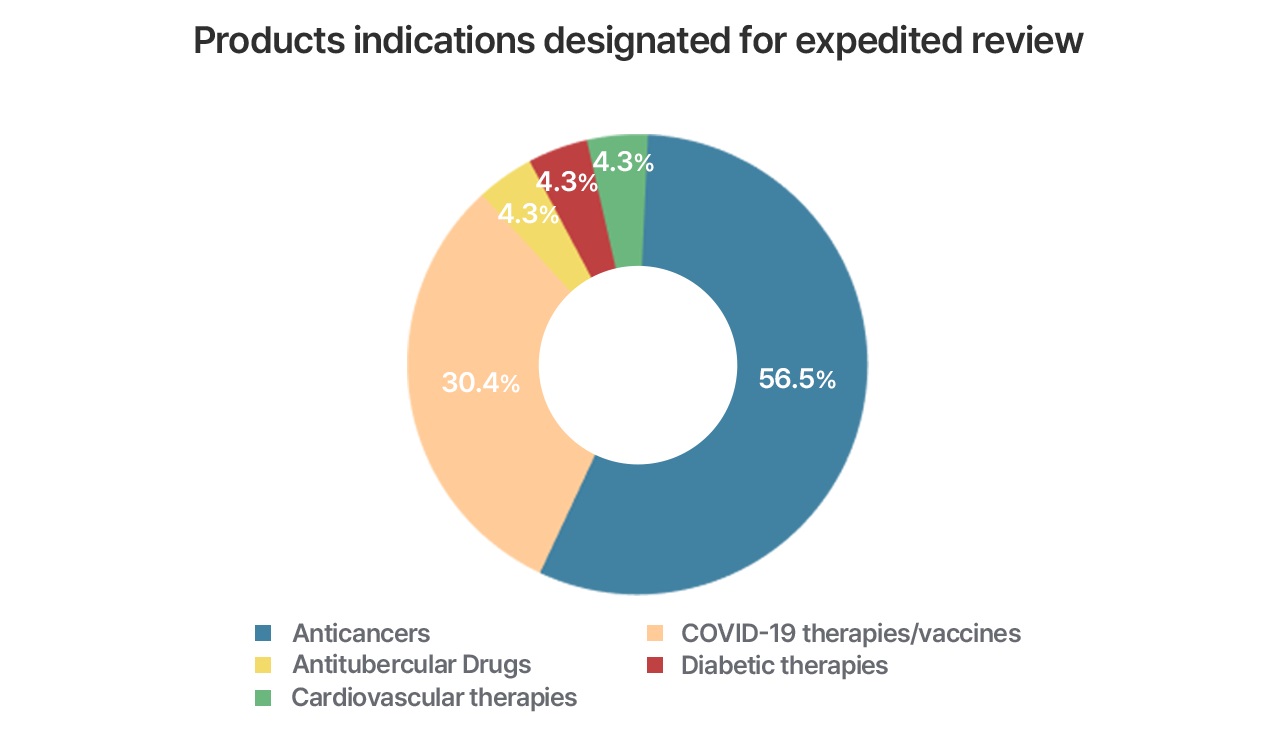
GIFT designation products
Visual guide of GIFT