The first approval of AI-based Cancer Diagnostics Software in Korea
- Registration Date 2020-06-29
- Hit 1116
The first AI-based software for cancer diagnosis approved in Korea will assist physicians in cancer diagnosis
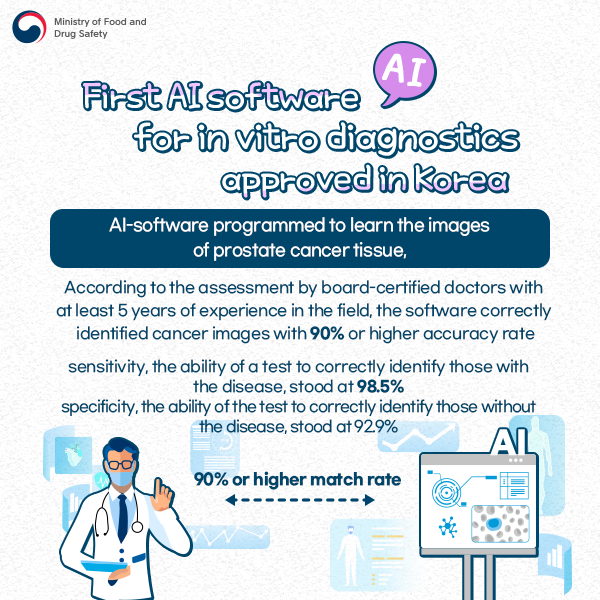
[First AI software for in-vitro diagnostics approved in Korea]
AI-software programmed to learn the images of prostate cancer tissue
According to the assessment by board-certified doctors with at least 5 years of experience in the field, the software correctly identified cancer images with 90% or higher accuracy rate.
Sensitivity, the ability of a test to correctly identify those with the disease, stood at 98.5%.
Specificity, the ability of the test to correctly identify those without the disease, stood at 92.9%.
(90% or higher match rate)
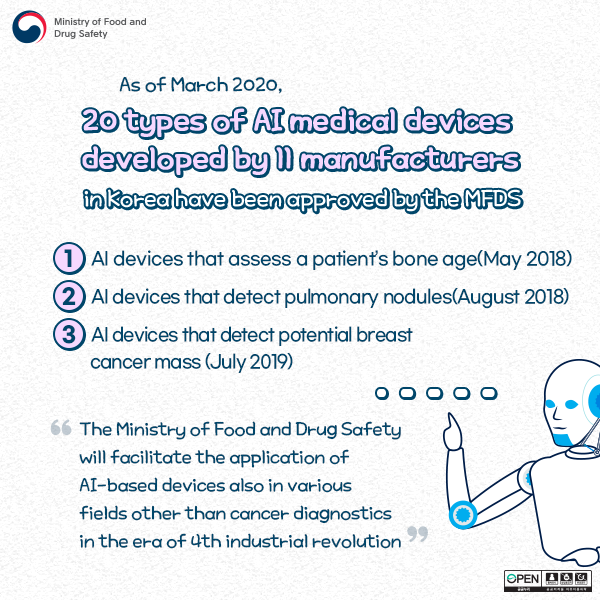
As of March 2020,
20 types of AI medical devices developed by 11 manufacturers in Korea have been approved by the MFDS.
1) AI devices that assess a patient's bone age (May 2018)
2) AI devices that detect pulmonary nodules (August 2018)
3) AI devices that detect potential breast cancer mass (July 2019)
The Ministry of Food and Drug Safety will facilitate the application of AI-based devices also in various fields other than cancer diagnostics in the era of 4th industrial revolution.
Please refer to the Press Release on April 3, 2020 uploaded on our website under MFDS News > News & Notice for more information concerning this card news.
[First AI software for in-vitro diagnostics approved in Korea]
AI-software programmed to learn the images of prostate cancer tissue
According to the assessment by board-certified doctors with at least 5 years of experience in the field, the software correctly identified cancer images with 90% or higher accuracy rate.
Sensitivity, the ability of a test to correctly identify those with the disease, stood at 98.5%.
Specificity, the ability of the test to correctly identify those without the disease, stood at 92.9%.
(90% or higher match rate)
As of March 2020,
20 types of AI medical devices developed by 11 manufacturers in Korea have been approved by the MFDS.
1) AI devices that assess a patient's bone age (May 2018)
2) AI devices that detect pulmonary nodules (August 2018)
3) AI devices that detect potential breast cancer mass (July 2019)
The Ministry of Food and Drug Safety will facilitate the application of AI-based devices also in various fields other than cancer diagnostics in the era of 4th industrial revolution.
Please refer to the Press Release on April 3, 2020 uploaded on our website under MFDS News > News & Notice for more information concerning this card news.
Division 국제협력담당관
Written by 배성명
Telephone 043-719-1559