Implementation of the Act on In Vitro Diagnostic Medical Devices (IVDs)
- Registration Date 2020-06-29
- Hit 1375
On May 1, 2020, "Act on In Vitro Diagnostic Medical Devices (IVDs)" will be implemented.
IVDs, such as COVID-19 test reagents, will be regulated according to the IVD classification system.
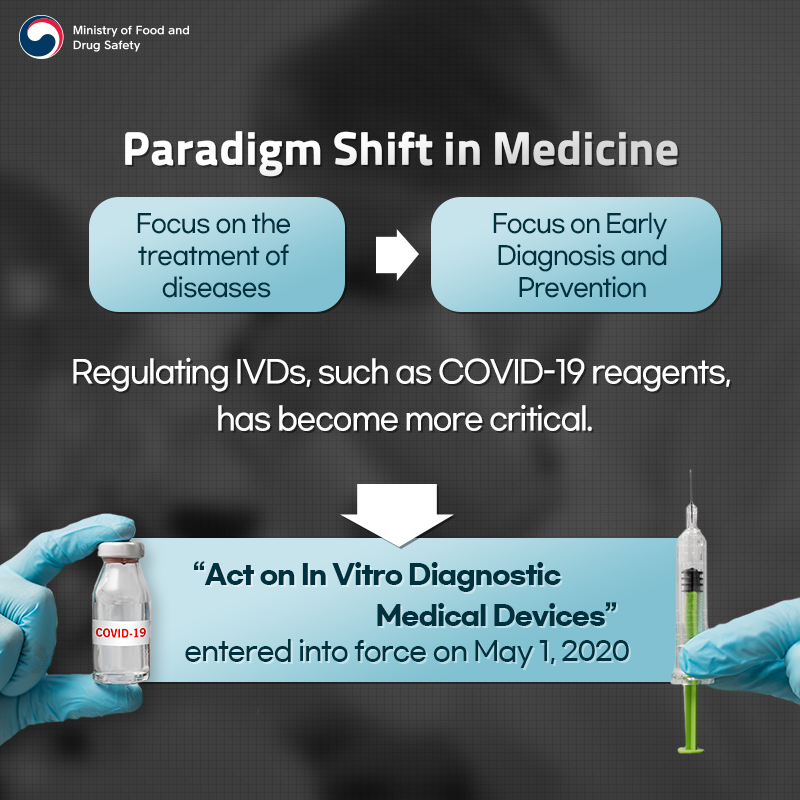
[Paradigm Shift in Medicine]
Focus on the treatment of diseases -> Focus on Early Diagnosis and Prevention
Regulating IVDs, such as COVID-19 reagents, has become more critical.
"Act on In Vitro Diagnostic Medical Devices" entered into force on May 1, 2020.
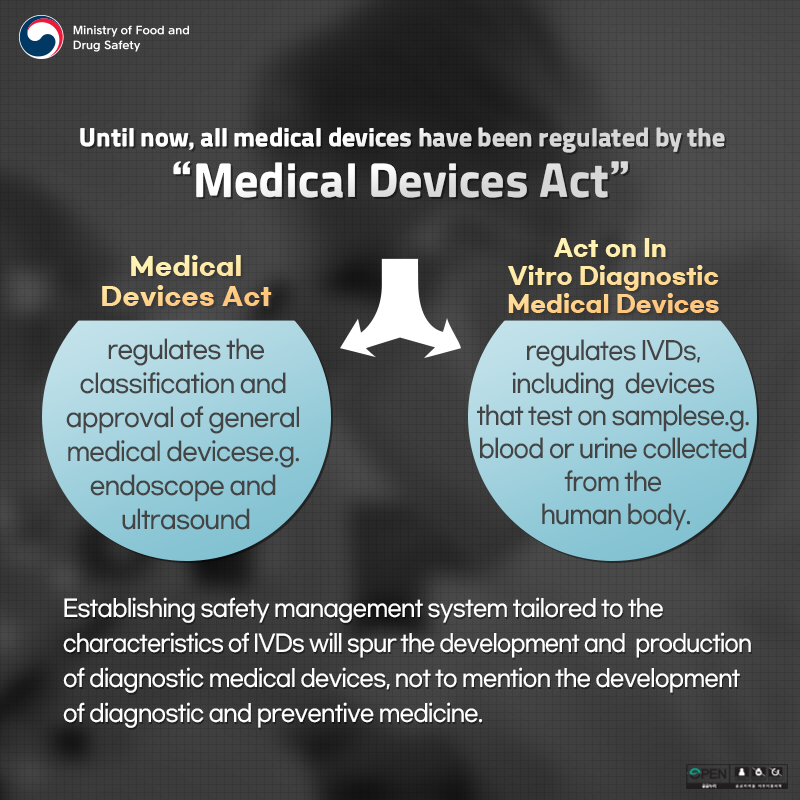
Until now, all medical devices have been regulated by the "Medical Devices Act"
Medical Devices Act regulates the classification and approval of general medical devices (e.g. endoscope and ultrasound)
Act on In Vitro Diagnostic Medical Devices regulates IVDs, including devices that test on samples (e.g. blood or urine collected from the human body)
Establishing safety management system tailored to the characteristics of IVDs will spur the development and production of diagnostic medical devices, not to mention the development of diagnostic and preventive medicine.
Please refer to the Press Release on May 1, 2020 uploaded on our website under MFDS News > News & Notice
for more information concerning this card news.
IVDs, such as COVID-19 test reagents, will be regulated according to the IVD classification system.
[Paradigm Shift in Medicine]
Focus on the treatment of diseases -> Focus on Early Diagnosis and Prevention
Regulating IVDs, such as COVID-19 reagents, has become more critical.
"Act on In Vitro Diagnostic Medical Devices" entered into force on May 1, 2020.
Until now, all medical devices have been regulated by the "Medical Devices Act"
Medical Devices Act regulates the classification and approval of general medical devices (e.g. endoscope and ultrasound)
Act on In Vitro Diagnostic Medical Devices regulates IVDs, including devices that test on samples (e.g. blood or urine collected from the human body)
Establishing safety management system tailored to the characteristics of IVDs will spur the development and production of diagnostic medical devices, not to mention the development of diagnostic and preventive medicine.
Please refer to the Press Release on May 1, 2020 uploaded on our website under MFDS News > News & Notice
for more information concerning this card news.
Division 국제협력담당관
Written by 배성명
Telephone 043-719-1559