The government regulatory burden of proof in food and drug sectors
- Registration Date 2020-08-10
- Hit 1332
The government regulatory burden of proof in food and drug sectors
Achievements made in the second half of 2019
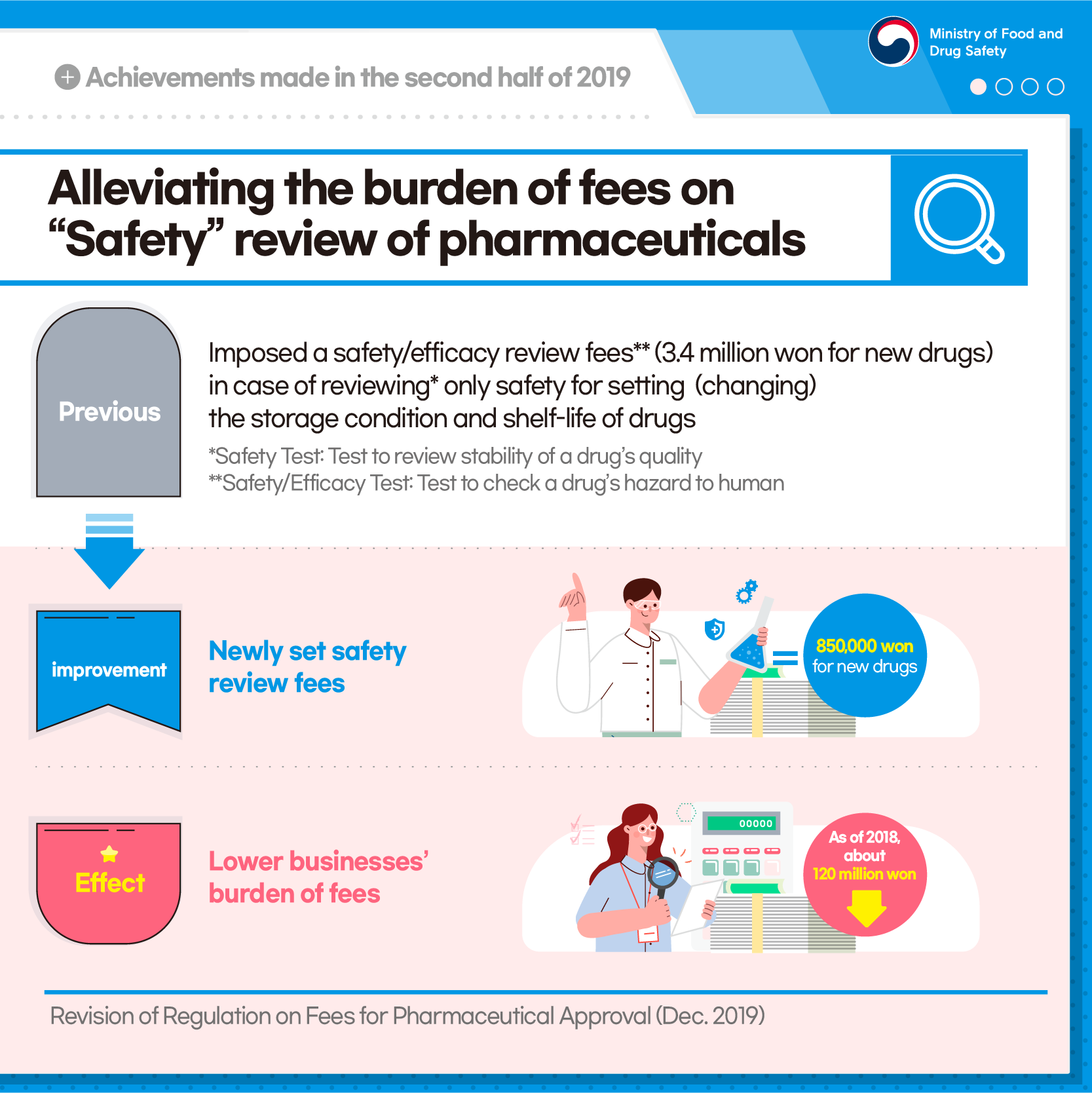
Alleviating the burden of fees on "Safety" review of pharmaceuticals
[Previous] Imposed a safety/efficacy review fees** (3.4 million won for new drugs) in case of reviewing* only safety for setting (changing) the storage condition and shelf-life of drugs
*Safety Test: Test to review stability of a drug's quality
**Safety/Efficacy Test: Test to check a drug's hazard to human
[Improvement] Newly set safety review fees
850,000 won for new drugs
[Effect] Lower businesses' burden of fees
As of 2018, about 120 million won down
Revision of Regulation on Fees for Pharmaceutical Approval (Dec. 2019)
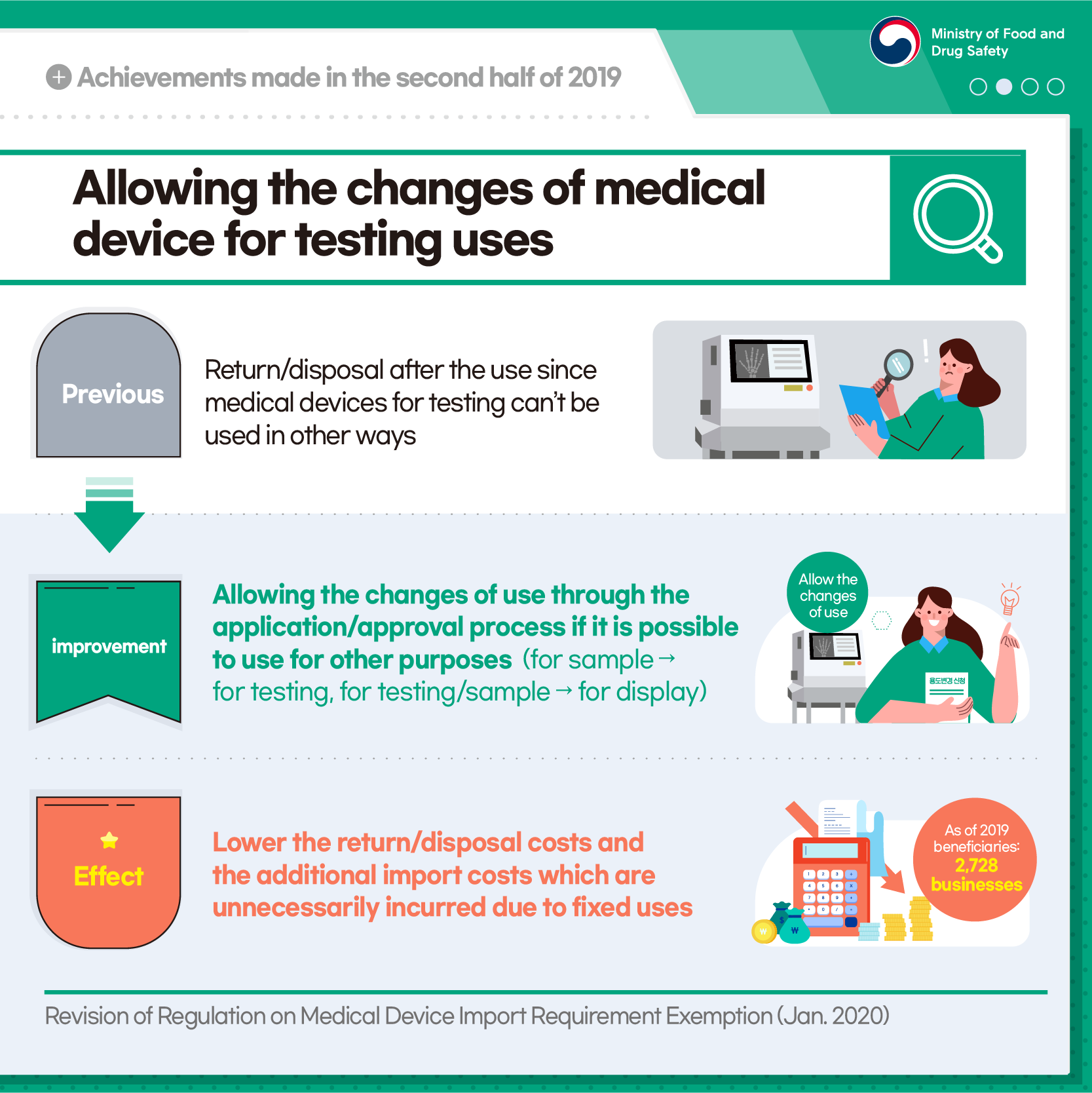
Allowing the changes of medical device for testing uses
[Previous] Return/disposal after the use since medical devices for testing can't be used in other ways
[Improvement] Allowing the changes of use through the application/approval process if it is possible to use for other purposes (for sample -> for testing, for testing/sample -> for display)
[Effect] Lower the return/disposal costs and the additional import costs which are unnecessarily incurred due to fixed uses
As of 2019 beneficiaries: 2,728 businesses
Revision of Regulation on Medical Device Import Requirement Exemption (Jan. 2020)
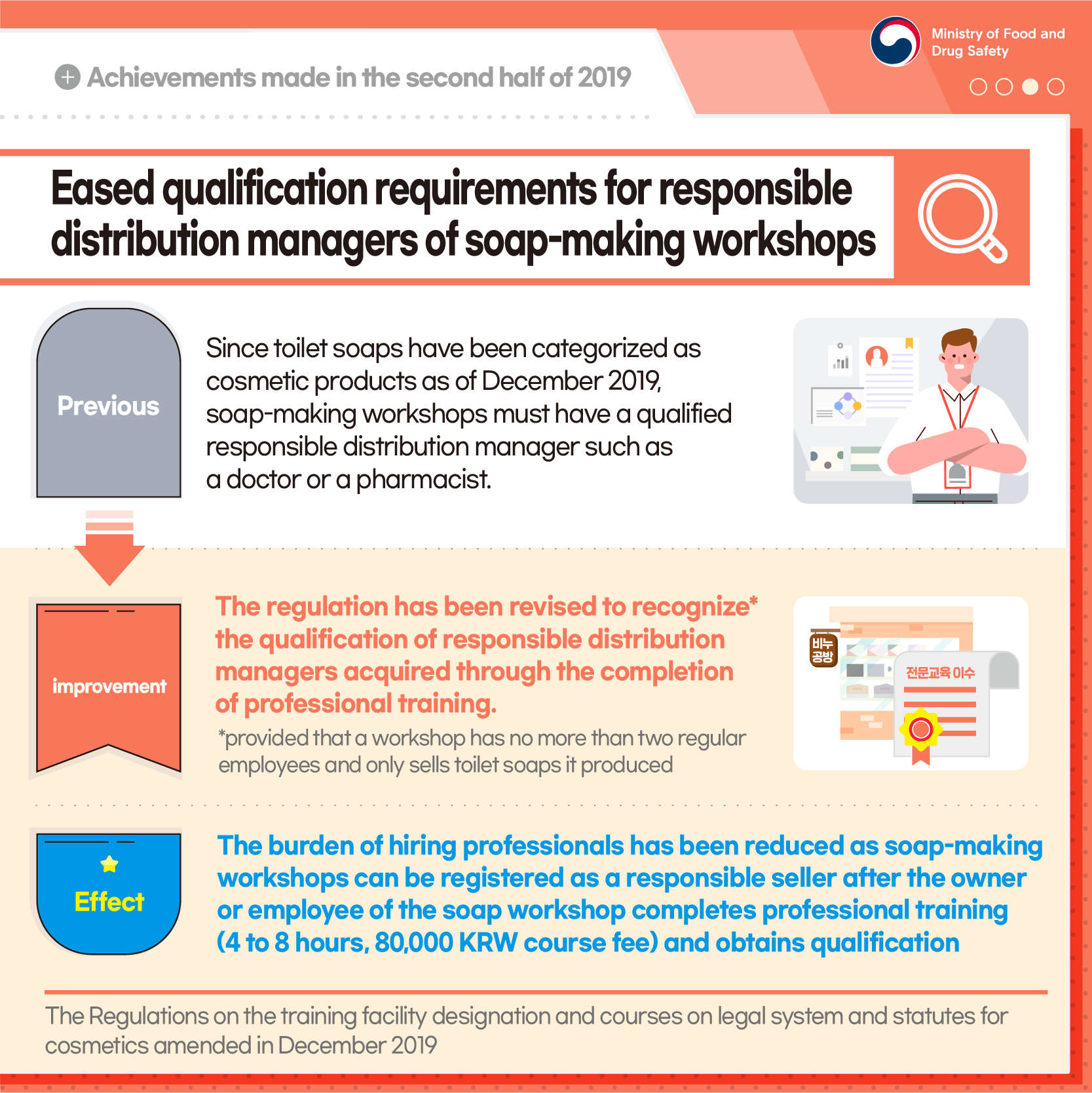
Eased qualification requirements for responsible distribution managers of soap-making workshops
[Previous] Since toilet soaps have been categorized as cosmetic products as of December 2019, soap-making workshops must have a qualified responsible distribution manager such as a doctor or a pharmacist.
[Improvement] The regulation has been revised to recognize* the qualification of responsible distribution managers acquired through the completion of professional training.
*provided that a workshop has no more than two regular employees and only sells toilet soaps it produced
[Effect] The burden of hiring professionals has been reduced as soap-making workshops can be registered as a responsible seller after the owner or employee of the soap workshop completes professional training (4 to 8 hours, 80,000 KRW course fee) and obtains qualification
The Regulations on the training facility designation and courses on legal system and statues for cosmetics amended in December 2019
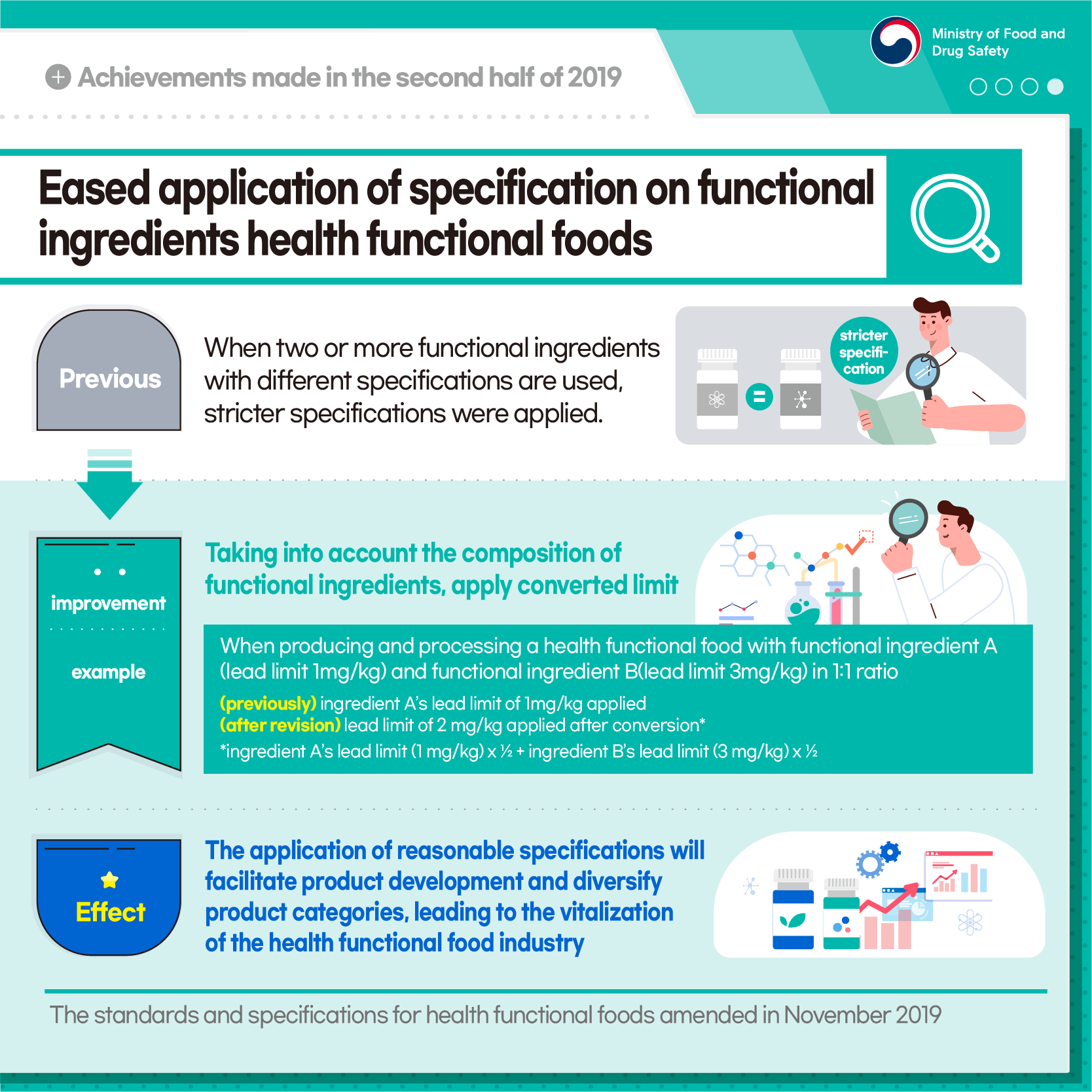
Eased application of specification on functional ingredients health functional foods
[Previous] When two or more functional ingredients with different specifications are used, stricter specifications were applied.
[Improvement] Taking into account the composition of functional ingredients, apply converted limit
[Example] When producing and processing a health functional food with functional ingredient A (lead limit 1 mg/kg) and functional ingredient B (lead limit 3 mg/kg) in 1:1 ratio
(previously) ingredient A's lead limit of 1 mg/kg applied
(after revision) lead limit of 2 mg/kg applied after conversion*
*ingredient A's lead limit (1 mg/kg) x 1/2 +_ingredient B's lead limit (3 mg/kg) x 1/2
[Effect] The application of reasonable specifications will facilitate product development and diversify product categories, leading to the vitalization of the health functional food industry
The Standards and Specifications for Health Functional Foods amended in Nov 2019
Achievements made in the second half of 2019
Alleviating the burden of fees on "Safety" review of pharmaceuticals
[Previous] Imposed a safety/efficacy review fees** (3.4 million won for new drugs) in case of reviewing* only safety for setting (changing) the storage condition and shelf-life of drugs
*Safety Test: Test to review stability of a drug's quality
**Safety/Efficacy Test: Test to check a drug's hazard to human
[Improvement] Newly set safety review fees
850,000 won for new drugs
[Effect] Lower businesses' burden of fees
As of 2018, about 120 million won down
Revision of Regulation on Fees for Pharmaceutical Approval (Dec. 2019)
Allowing the changes of medical device for testing uses
[Previous] Return/disposal after the use since medical devices for testing can't be used in other ways
[Improvement] Allowing the changes of use through the application/approval process if it is possible to use for other purposes (for sample -> for testing, for testing/sample -> for display)
[Effect] Lower the return/disposal costs and the additional import costs which are unnecessarily incurred due to fixed uses
As of 2019 beneficiaries: 2,728 businesses
Revision of Regulation on Medical Device Import Requirement Exemption (Jan. 2020)
Eased qualification requirements for responsible distribution managers of soap-making workshops
[Previous] Since toilet soaps have been categorized as cosmetic products as of December 2019, soap-making workshops must have a qualified responsible distribution manager such as a doctor or a pharmacist.
[Improvement] The regulation has been revised to recognize* the qualification of responsible distribution managers acquired through the completion of professional training.
*provided that a workshop has no more than two regular employees and only sells toilet soaps it produced
[Effect] The burden of hiring professionals has been reduced as soap-making workshops can be registered as a responsible seller after the owner or employee of the soap workshop completes professional training (4 to 8 hours, 80,000 KRW course fee) and obtains qualification
The Regulations on the training facility designation and courses on legal system and statues for cosmetics amended in December 2019
Eased application of specification on functional ingredients health functional foods
[Previous] When two or more functional ingredients with different specifications are used, stricter specifications were applied.
[Improvement] Taking into account the composition of functional ingredients, apply converted limit
[Example] When producing and processing a health functional food with functional ingredient A (lead limit 1 mg/kg) and functional ingredient B (lead limit 3 mg/kg) in 1:1 ratio
(previously) ingredient A's lead limit of 1 mg/kg applied
(after revision) lead limit of 2 mg/kg applied after conversion*
*ingredient A's lead limit (1 mg/kg) x 1/2 +_ingredient B's lead limit (3 mg/kg) x 1/2
[Effect] The application of reasonable specifications will facilitate product development and diversify product categories, leading to the vitalization of the health functional food industry
The Standards and Specifications for Health Functional Foods amended in Nov 2019
Division 국제협력담당관
Written by 배성명
Telephone 043-719-1559