- Registration Date 2024-05-29
- Hit 4982
|
2024/1333 |
21.5.2024 |
COMMISSION IMPLEMENTING REGULATION (EU) 2024/1333
of 17 May 2024
amending and correcting Annex III to Implementing Regulation (EU) 2020/2235 as regards model certificates for the entry into the Union of live fish, live crustaceans and products of animal origin from those animals and certain fishery products, intended for human consumption
(Text with EEA relevance)
THE EUROPEAN COMMISSION,
Having regard to Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 on laying down specific hygiene rules for food of animal origin (1), and in particular Article 7(2), point (a), thereof,
Having regard to Regulation (EU) 2016/429 of the European Parliament and of the Council of 9 March 2016 on transmissible animal diseases and amending and repealing certain acts in the area of animal health (‘Animal Health Law’) (2), and in particular Articles 238(3) and 239(3) thereof,
Having regard to Regulation (EU) 2017/625 of the European Parliament and of the Council of 15 March 2017 on official controls and other official activities performed to ensure the application of food and feed law, rules on animal health and welfare, plant health and plant protection products, amending Regulations (EC) No 999/2001, (EC) No 396/2005, (EC) No 1069/2009, (EC) No 1107/2009, (EU) No 1151/2012, (EU) No 652/2014, (EU) 2016/429 and (EU) 2016/2031 of the European Parliament and of the Council, Council Regulations (EC) No 1/2005 and (EC) No 1099/2009 and Council Directives 98/58/EC, 1999/74/EC, 2007/43/EC, 2008/119/EC and 2008/120/EC and repealing Regulations (EC) No 854/2004 and (EC) No 882/2004 of the European Parliament and of the Council, Council Directives 89/608/EEC, 89/662/EEC, 90/425/EEC, 91/496/EEC, 96/23/EC, 96/93/EC and 97/78/EC and Council Decision 92/438/EEC (Official Controls Regulation) (3), and in particular Article 90, first paragraph, point (a) and Article 126(3) thereof,
Whereas:
|
(1) |
Commission Implementing Regulation (EU) 2020/2235 (4) lays down rules regarding animal health certificates provided for in Regulation (EU) 2016/429, official certificates and attestations provided for in Regulation (EU) 2017/625, and animal health/official certificates based on both those Regulations, required, among others, for the entry into the Union of certain consignments of animals and goods. |
|
(2) |
Chapter 28 (model FISH-CRUST-HC) of Annex III to Implementing Regulation (EU) 2020/2235 sets out the model animal health/official certificate for the entry into the Union of live fish, live crustaceans and products of animal origin from those animals intended for human consumption. Commission Implementing Regulation (EU) 2023/2744 (5) replaced that Chapter. In points II.2.6.2(i) and (iii) of the replaced Chapter 28, Implementing Regulation (EU) 2023/2744 contained two excess notes. In the interest of clarity, those notes should be deleted. |
|
(3) |
Chapter 29 (model EU-FISH) of Annex III to Implementing Regulation (EU) 2020/2235 sets out the model official certificate for the entry into the Union of fishery products intended for human consumption caught by vessels flying the flag of a Member State and transferred in third countries with or without storage. Implementing Regulation (EU) 2023/2744 deleted Part II of that Chapter erroneously. Part II of that Chapter should therefore be restored, and, for the sake of clarity, the order of the points be modified. |
|
(4) |
Council Directive 96/23/EC (6) was repealed and its provisions regarding entry into the Union laid down in Article 29 of that Directive were incorporated in Commission Delegated Regulation (EU) 2022/2292 (7). Moreover, Commission Decision 2011/163/EU (8) was repealed and its provisions were incorporated in Commission Implementing Regulation (EU) 2021/405 (9). It is therefore necessary to adapt the references to that Directive and that Decision in Chapter 29, Part II, of Annex III to Implementing Regulation (EU) 2020/2235 accordingly in the text to be restored. |
|
(5) |
As fishery products from wild catch are excluded from the application of the requirements laid down in Articles 6 to 12 of Delegated Regulation (EU) 2022/2292, a separate certification option should be introduced for those products in Chapter 29, Part II, of Annex III to Implementing Regulation (EU) 2020/2235. In relation to contaminants, that certification option should refer to compliance with Commission Regulation (EU) 2023/915 (10), and, in relation to pesticide residues, to compliance with Regulation (EC) No 396/2005 of the European Parliament and of the Council (11). |
|
(6) |
Implementing Regulation (EU) 2020/2235 should therefore be amended and corrected accordingly. |
|
(7) |
As the amendments made to Implementing Regulation (EU) 2020/2235 by Implementing Regulation (EU) 2023/2744 entered into force on 4 January 2024, in the interest of legal certainty and to facilitate trade, the amendments and corrections made to Implementing Regulation (EU) 2020/2235 by this Regulation should take effect as a matter of urgency. The urgent entry into force of the amendments and corrections is without prejudice to the fact that, in accordance with Article 2 of Implementing Regulation (EU) 2023/2744, until 15 September 2024, consignments of live fish, live crustaceans and products of animal origin from those animals and certain fishery products accompanied by the appropriate animal health/official certificate or official certificate issued in accordance with the models set out in Chapters 28 and 29 of Annex III to Implementing Regulation (EU) 2020/2235, as applicable before the amendments made to that Implementing Regulation by Implementing Regulation (EU) 2023/2744, continue to be authorised for the entry into the Union provided that the certificate was issued no later than 15 June 2024. |
|
(8) |
The measures provided for in this Regulation are in accordance with the opinion of the Standing Committee on Plants, Animals, Food and Feed, |
HAS ADOPTED THIS REGULATION:
Article 1
Annex III to Implementing Regulation (EU) 2020/2235 is amended and corrected in accordance with the Annex to this Regulation.
Article 2
This Regulation shall enter into force on the day following that of its publication in the Official Journal of the European Union.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
Done at Brussels, 17 May 2024.
For the Commission
The President
Ursula VON DER LEYEN
(1) OJ L 139, 30.4.2004, p. 55, ELI: http://data.europa.eu/eli/reg/2004/853/oj.
(2) OJ L 84, 31.3.2016, p. 1, ELI: http://data.europa.eu/eli/reg/2016/429/oj.
(3) OJ L 95, 7.4.2017, p. 1, ELI: http://data.europa.eu/eli/reg/2017/625/oj.
(4) Commission Implementing Regulation (EU) 2020/2235 of 16 December 2020 laying down rules for the application of Regulations (EU) 2016/429 and (EU) No 2017/625 of the European Parliament and of the Council as regards model animal health certificates, model official certificates and model animal health/official certificates, for the entry into the Union and movements within the Union of consignments of certain categories of animals and goods, official certification regarding such certificates and repealing Regulation (EC) No 599/2004, Implementing Regulations (EU) No 636/2014 and (EU) No 2019/628, Directive 98/68/EC and Decisions 2000/572/EC, 2003/779/EC and 2007/240/EC (OJ L 442, 30.12.2020, p. 1, ELI: http://data.europa.eu/eli/reg_impl/2020/2235/oj).
(5) Commission Implementing Regulation (EU) 2023/2744 of 20 November 2023 amending Implementing Regulation (EU) 2020/2235 as regards model animal health certificates, model official certificates, model animal health/official certificates and private attestation, for the entry into the Union or transit through the Union of consignments of certain categories of animals and goods, and official certification regarding such certificates (OJ L 2023/2744, 15.12.2023, ELI: http://data.europa.eu/eli/reg_impl/2023/2744/oj).
(6) Council Directive 96/23/EC of 29 April 1996 on measures to monitor certain substances and residues thereof in live animals and animal products and repealing Directives 85/358/EEC and 86/469/EEC and Decisions 89/187/EEC and 91/664/EEC (OJ L 125, 23.5.1996, p. 10, ELI: http://data.europa.eu/eli/dir/1996/23/oj).
(7) Commission Delegated Regulation (EU) 2022/2292 of 6 September 2022 supplementing Regulation (EU) 2017/625 of the European Parliament and of the Council with regard to requirements for the entry into the Union of consignments of food-producing animals and certain goods intended for human consumption (OJ L 304, 24.11.2022, p. 1, ELI: http://data.europa.eu/eli/reg_del/2022/2292/oj).
(8) Commission Decision 2011/163/EU of 16 March 2011 on the approval of plans submitted by third countries in accordance with Article 29 of Council Directive 96/23/EC (OJ L 70, 17.3.2011, p. 40, ELI: http://data.europa.eu/eli/dec/2011/163(1)/oj).
(9) Commission Implementing Regulation (EU) 2021/405 of 24 March 2021 laying down the lists of third countries or regions thereof authorised for the entry into the Union of certain animals and goods intended for human consumption in accordance with Regulation (EU) 2017/625 of the European Parliament and of the Council (OJ L 114, 31.3.2021, p. 118, ELI: http://data.europa.eu/eli/reg_impl/2021/405/oj).
(10) Commission Regulation (EU) 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing Regulation (EC) No 1881/2006 (OJ L 119, 5.5.2023, p. 103, ELI: http://data.europa.eu/eli/reg/2023/915/oj).
(11) Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC (OJ L 70, 16.3.2005, p. 1, ELI: http://data.europa.eu/eli/reg/2005/396/oj).
ANNEX
Annex III to Implementing Regulation (EU) 2020/2235 is amended and corrected as follows:
|
(1) |
in Chapter 28, point II.2.6.2 is replaced by the following:
|
|
(2) |
in Chapter 29, the following is added: ‘  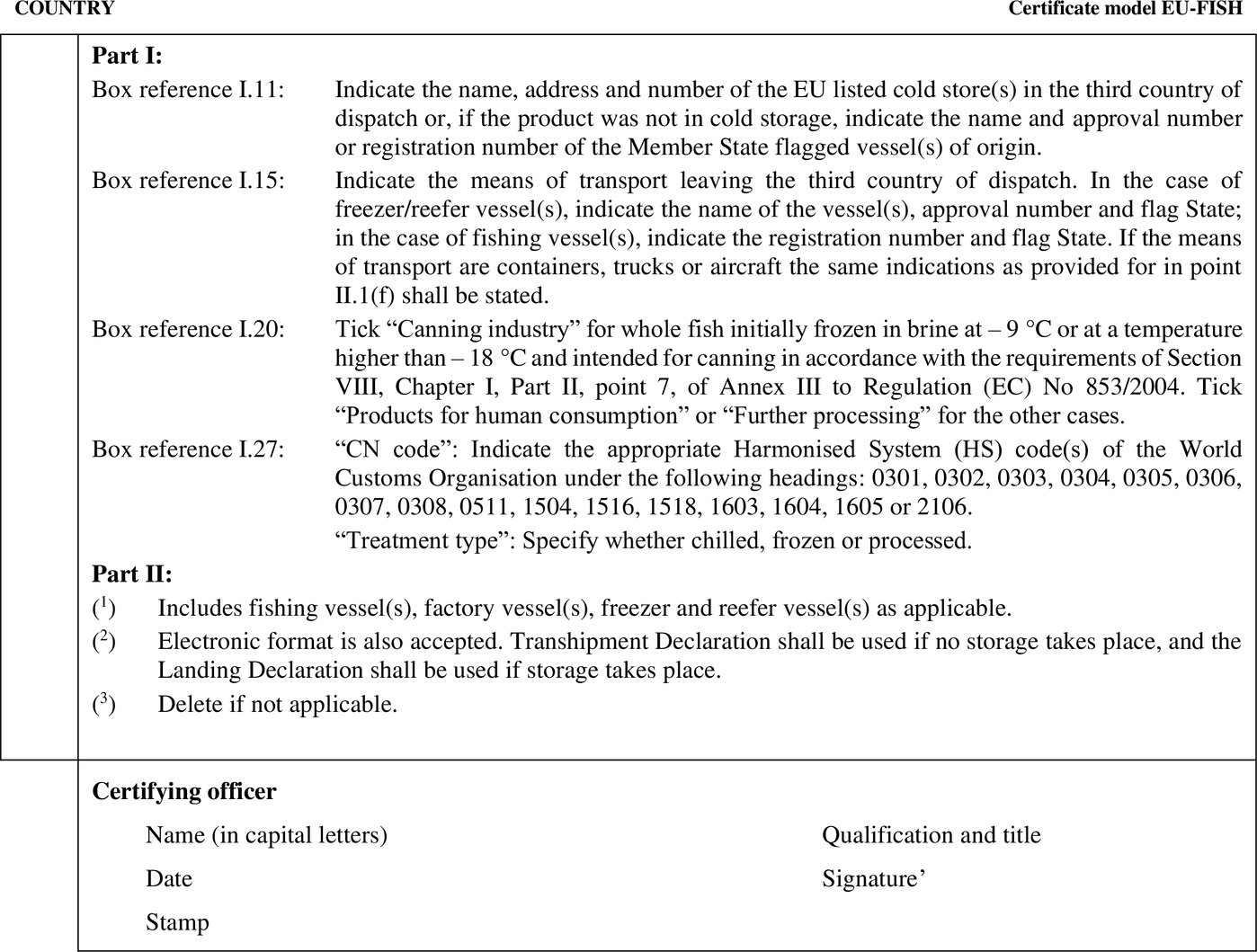 |
ELI: http://data.europa.eu/eli/reg_impl/2024/1333/oj
ISSN 1977-0677 (electronic edition)
Division Risk Information DIvision
Written by Risk Information DIvision

