- Registration Date 2016-10-14
- Hit 12070
|
Company Information |
|
|
1. Name |
Janssen Vaccines Corp. |
|
2. Website Address |
|
|
3. Location |
(Songdo-dong)23, Harmony-ro 303beon-gil, Yeonsu-gu, Incheon, Korea |
|
4. Contacts |
+82-32-290-8400 |
|
5. References |
|
|
Pharmaceutical Product Information |
|
|
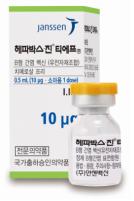
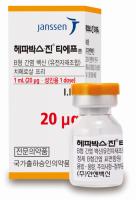
1. Brand Name |
Hepavax-Gene® TF inj. |
|
2. Active Ingredient |
Purified hepatitis B surface antigen (host cell: Hansenular polymorpha YU305, vector: pGM834 (FMD promoter) |
|
3. Indication |
Immunization against hepatitis B |
|
4. Information |
1.0 mL of this product (1.0 mL; 20 mg) should be intramuscularly administered one time to adults. To neonates, infants and children, 0.5 mL (10 mg) should be intramuscularly administered one time. 1. Standard vaccination course: 1st vaccination: at the elected date 2nd vaccination: 1 month after the 1st vaccination 3rd vaccination: 6 months after the 1st vaccination However, for those who need fast protection effect, such as those at high risk of exposure to hepatitis B virus or those who cannot follow the standard vaccination schedule, it is recommended to intramuscularly administer this product three times at 1-month interval. |
|
5. Images |
|
|
harmaceutical Product Information |
|
|
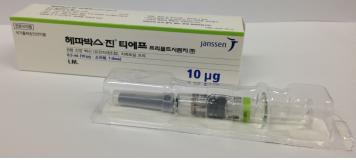
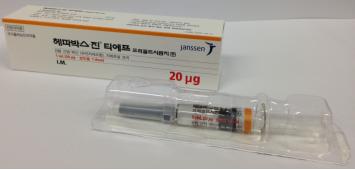
3. Brand Name |
Hepavax-Gene® TF Prefilled Syringe inj. |
|
4. Active Ingredient |
Purified hepatitis B surface antigen (host cell: Hansenular polymorpha YU305, vector: pGM834 (FMD promoter) |
|
3. Indication |
Immunization against hepatitis B |
|
4. Information |
1.0 mL of this product (1.0 mL; 20 mg) should be intramuscularly administered one time to adults. To neonates, infants and children, 0.5 mL (10 mg) should be intramuscularly administered one time. 1. Standard vaccination course: 1st vaccination: at the elected date 2nd vaccination: 1 month after the 1st vaccination 3rd vaccination: 6 months after the 1st vaccination However, for those who need fast protection effect, such as those at high risk of exposure to hepatitis B virus or those who cannot follow the standard vaccination schedule, it is recommended to intramuscularly administer this product three times at 1-month interval. |
|
5. Images |
|
Division
Written by 전형옥