- Registration Date 2016-10-14
- Hit 7102
|
Company Information |
|
|
1. Name |
SEWON CELLONTECH CO., LTD. |
|
2. Website Address |
|
|
3. Location |
Wooyoung Techno Center, 144, Achasan-ro, Seongdong-gu Seoul, 04783, Rep. of KOREA |
|
4. Contacts |
Email: contact@swcell.com |
|
5. References |
|
|
Pharmaceutical Product Information |
|
|
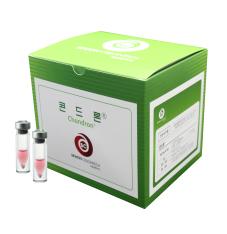
1. Brand Name |
ChondronTM |
|
2. Active Ingredient |
Cultured Autologous Chondrocytes |
|
3. Indication |
Focal Cartilage Defect of Knee |
|
4. Information |
ChondronTM is cell therapy product for autologous chondrocyte implantation (ACI). One to six vials, which contains more than 12 million chondrocytes per vial, are provided for implantation. ChondronTM can be also used with bio-compatible material (fibrin glue). |
|
5. Images |
|
|
Pharmaceutical Product Information |
|
|
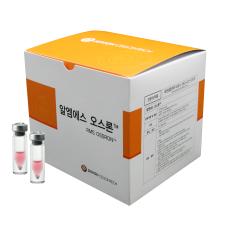
1. Brand Name |
RMS OssronTM |
|
2. Active Ingredient |
Cultured Autologous Osteoblasts |
|
3. Indication |
Focal bone formation |
|
4. Information |
RMS OssronTM is cell therapy product for autologous bone marrow cell implantation. One to six vials, which contains more than 12 million osteoblasts per vial, are provided for implantation. RMS OssronTM can be surgically implanted with bio-compatible material (fibrin glue). |
|
5. Images |
|
Division
Written by 전형옥