- Registration Date 2016-10-14
- Hit 5386
|
Company Information |
|
|
1. Name |
Green Cross Cell Corp. |
|
2. Website Address |
www.greencrosscell.com |
|
3. Location |
6th floor, 278, Beotkkot-ro, Geumcheon-gu, Seoul, Korea, 08511 |
|
4. Contacts |
Tel: +82-2-2101-0600 Fax: +82-2-2101-0601 |
|
5. References |
|
|
Pharmaceutical Product Information |
|
|
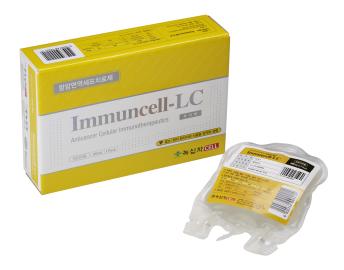
1. Brand Name |
Immuncell-LC |
|
2. Active Ingredient |
Autologous activated T-cell |
|
3. Indication |
Liver cancer(hepatocellular carcinoma) |
|
4. Information |
Immuncell-LC only uses immune cells of individual patients and can enhance patients' quality of life with virtually no pain or side effects. Received approval for the hepatocellular carcinoma immunotherapy ‘Immuncell-LC’ from MFDS in 2007. Completed phase Ⅲ clinical trials for hepatocellular carcinoma and glioblastoma in korea.
① Adjuvant Immunotherapy With Autologous Cytokine-Induced Killer Cells for Hepatocellular Carcinoma(Gastroenterology, 2015) ② Phase III randomized trial of autologous cytokine-induced killer cell immunotherapy for newly diagnosed glioblastoma in korea(Oncotarget, 2016) ③ Phase II clinical trial of ex vivo‑expanded cytokine‑induced killer cells therapy in advanced pancreatic cancer(Cancer Immunology Immunotherapy, 2014)
|
|
5. Images |
|
Division
Written by 전형옥