- Registration Date 2016-03-24
- Hit 18149
|
Company Information |
|
|
1. Name |
Hugel, Inc. |
|
2. Website Address |
|
|
3. Location |
● HQ/ Plant 1 (Sinbuk): 61-20, Sinbuk-ro, Sinbuk-eup, Chuncheon-si, Gangwon-do 24206, Korea ● Plant 2 (Geodu): 23, Geodudanji 1-gil, Dongnae-myeon, Chuncheon-si, Gangwon-do 24398, Korea ● Seoul Office: 17F, Seongwon Tower, 514, Seolleung-ro, Gangnam-gu, Seoul 06162, Korea |
|
4. Contacts |
● HQ/ Plant 1 (Sinbuk): +82 33 255 3882 ● Plant 2 (Geodu): +82 33 815 5200 ● Seoul Office: +82 2 6966 1600 |
|
5. References |
● Attachment 1 (50unit manual) ● Attachment 2 (100unit manual) ● Attachment 3 (200unit manual) ● Attachment 4 (150unit manual) |
|
Pharmaceutical Product Information |
|
|
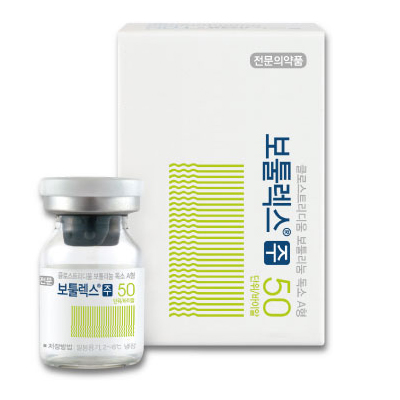
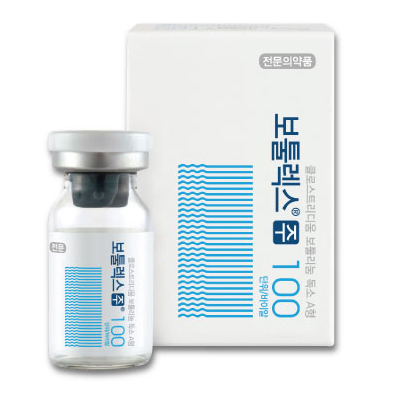
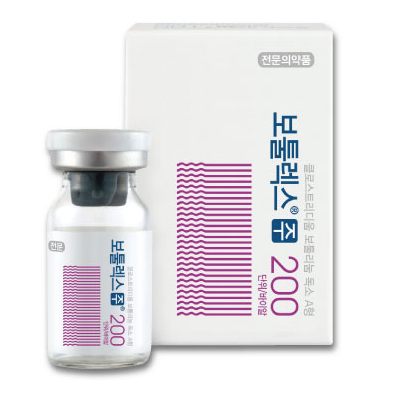
1. Brand name |
Botulax® 50U, Botulax® 100U, Botulax® 200U |
|
2. Active Ingredient |
Clostridium Botulinum Toxin Type A Strain: Clostridium Botulinum CBFC26 |
|
3. Indication |
1. Benign essential blepharospasm in adult patients, 18 years age or above 2. Temporary improvement of serious glabellar wrinkles ranging from moderate to severe associated with corrugators muscle and/or procerus muscle activities in adults aged between 18 and 65 |
|
4. Information |
● Since 2009, “Botulax” has been marketed in 22 countries, including Korea, Japan, Thailand, Vietnam, Philippines, Chile, Peru, Uruguay, Panama, Kuwait, etc. |
|
5. Images |
|
|
Pharmaceutical Product Information |
|
|
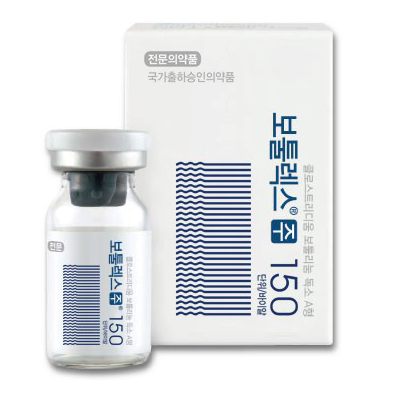
1. Brand name |
Botulax® 150U |
|
2. Active Ingredient |
Clostridium Botulinum Toxin Type A Strain: Clostridium Botulinum CBFC26 |
|
3. Indication |
1. Benign essential blepharospasm in adult patients, 18 years age or above 2. Temporary improvement of serious glabellar wrinkles ranging from moderate to severe associated with corrugators muscle and/or procerus muscle activities in adults aged between 18 and 65 3. Muscle spasticity : Upper limb spasticity associated with stroke in patients aged 20 or older 4. Treatment of equinus foot deformity due to spasticity in pediatric cerebral palsy patients aged 2 years or older
|
|
4. Images |
|
Division
Written by 전형옥